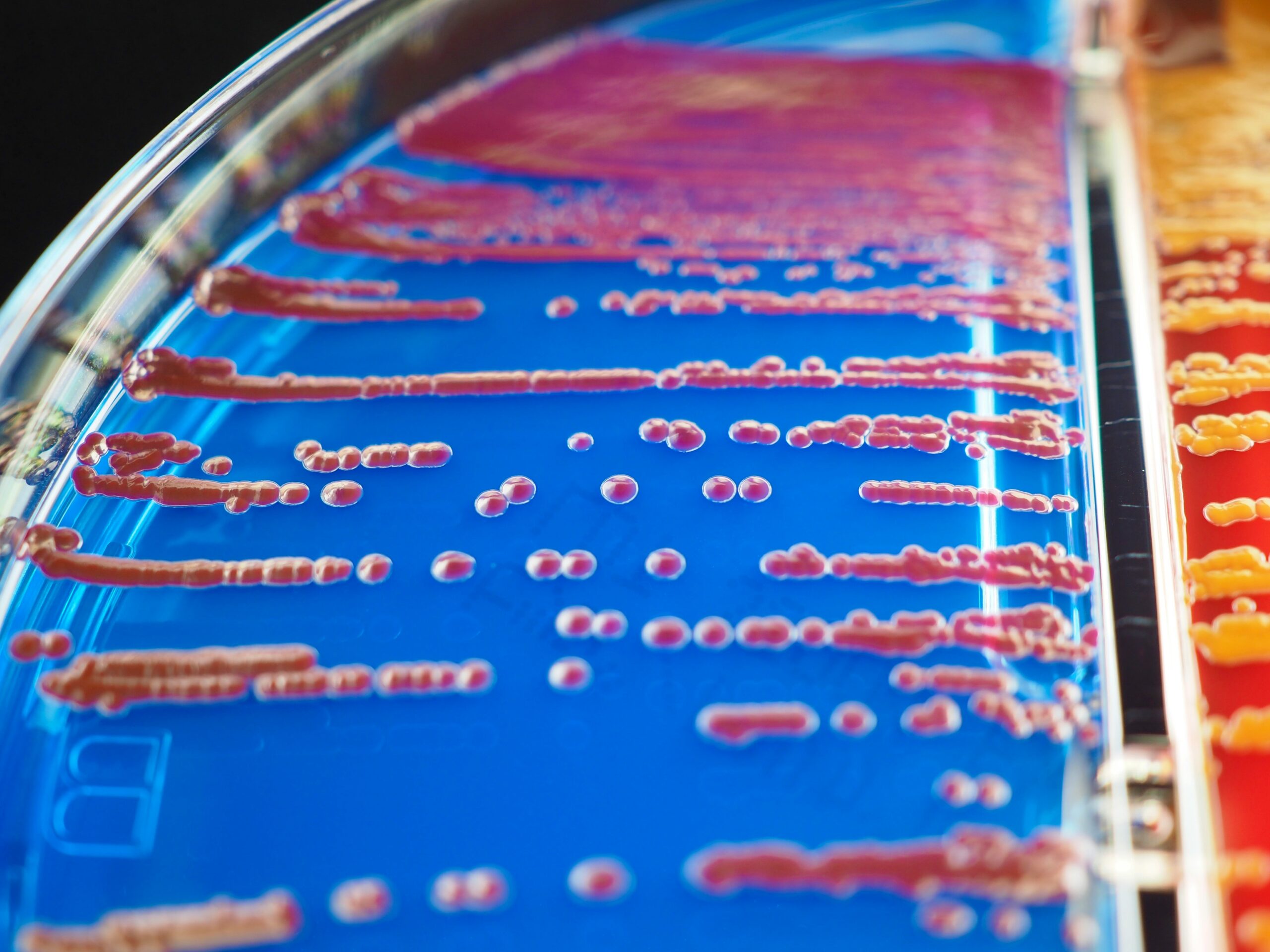
Agar is used in microbiology in order to identify bacteria and fungi and is either used in clinical settings for patient diagnosis or in research institutions. It can be found lining the bottom of petri dishes and is known as an agar plate in that form. Agar can also be found in glass tubes and is referred to as a slant. There are many types of agar which can be used, but we will focus here on some of the more common ones that can be found inside of a clinical laboratory.
How Are Agar Plates Used
Agar plates are used to grow cultures of microorganisms, both bacteria and fungi. Various specimens like throat swabs, genital swabs, wound swabs, stool, urine and sputum are rubbed onto a plate and spread out with an inoculation loop. This dilution of organism allows for isolation and results in individual colonies which can be used for further testing and identification. The most common technique to achieve isolation is the 4-Quadrant Streak, although the Colony Count technique is often used for urine specimens. There are different types of plates which perform special functions like being selective or differential. Selective plates will have inhibitors that keep certain bacteria from growing, for example, if you only want gram positive bacteria like Strep or Staph to grow. Whereas with Differential media, the agar will have additives like color indicators which can differentiate between different types of bacteria on the same plate due to color change.
How To Make Agar Plates
To give a better idea of what an agar plate is and how the various types are differentiated, let us first discuss how they are made. There are companies like Becton Dickinson that produce and sell these plates to medical and research institutions. This is very convenient, but costs more money. This is why some places choose to make their own. But with the exception of a handful of colleges and universities, making it homemade is rarely done anymore nowadays. The process of making agar is relatively simple and is similar to making Jello. First you get some agar powder and you mix it with water until it dissolves. Then the agar solution is boiled in order to sterilize it. Once sterilized, the solution is poured into sterile plates and allowed to cool and solidify before being packaged. Depending on what type of agar it is, certain special ingredients will need to be added to the agar as well, such as soy, sheep blood, peptones, salt, color indicators and more.
Blood Agar
The Blood Agar or Sheep Blood Agar (SBA) is the most common agar that is used in the laboratory. The reason for the name is due to the addition of 5% sheep blood which helps gives it its red color and assists with visualization of hemolysis, or lysis of the red blood cells. This defibrinated sheep blood is added to Trypticase Soy Agar and is known as TSA Agar with 5% Sheep Blood. Without the addition of the sheep blood, there are enough nutrients for many species of bacteria to grow, however hemolysis will not possible. Hemolysis is a huge part of differentiating different bacteria, which is why TSA plates are supplemented with sheep blood more often than not.
There are 3 types of hemolysis which can be observed on blood agar:
Beta Hemolysis
This can be seen when certain bacteria have enzymes which lyse the blood cells and cause a “halo” like appearance around each colony. Organisms like Staphylococcus aureus and Escherichia coli are examples of bacteria which can cause beta hemolysis on blood agar.
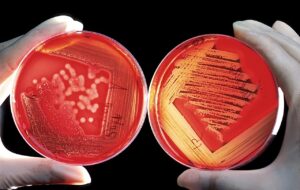
Alpha Hemolysis
As opposed to beta hemolysis where there is a complete clearing of the cells, alpha hemolysis is characterized by a partial clearing of cells, often seen as a green discoloration around each colony. Streptococcus pneumoniae is a good example of an organism which causes alpha hemolysis on blood agar.
Gamma Hemolysis
This is the total absence of hemolysis of the blood cells, seen as the absence of a clear halo or green discoloration surrounding the colony, as in beta and alpha hemolysis. Staphylococcus epidermidis is a common organism which exhibits gamma hemolysis on blood agar.
Double Zone Hemolysis
Some organisms have what is known as a double zone of hemolysis on blood agar. This is when there is an inner zone of beta hemolysis and an outer zone of alpha hemolysis around the colony. Though not a major category of hemolysis, it is still worth noting that this may be seen with certain strains.
Blood agar also contains other ingredients including casein, soybean meal, sodium chloride and growth factors. Its high protein nature provides plentiful supply of nitrogen and allows for a nutritious environment for fastidious bacteria to grow.
Blood agars are part of the media selection panel for virtually all types of specimens including wound, urine, stool, throat, respiratory and genital.
Chocolate Agar
Chocolate Agar is an enrichment agar which assists with the cultivation of “fastidious” bacteria like Haemophilus and Neisseria.
Chocolate agar gets its name due to its brownish hue which resembles the color of chocolate. Like the blood agar, chocolate agar also contains blood, but it is heated up until the cells have lysed, giving it the characteristic brown color.
The agar base contains casein and meat peptones which provide nitrogen. Hemoglobin is added which provides X factor or (Hemin) for the growth of Haemophilus species. IsoVitaleX provides V factor or (NAD) for Haemophilus species. IsoVitaleX also provides vitamins, amino acids, co-enzymes, dextrose, ferric ion and other factors which improve the growth of pathogenic Neisseria. Common organisms which are cultivated on chocolate agar are Haemophilus influenzae, Haemophilus parainfluenzae, Neisseria gonorrhoeae and Neisseria meningitidis.
Modified Thayer Martin (MTM) Agar
Modified Thayer-Martin (MTM) Agar is an enriched medium for the selective isolation and cultivation of Neisseria species from specimens containing mixed flora from the throat, vagina, rectum, and urethra.
This agar started out as Thayer Martin Agar and consisted of Chocolate Agar supplemented with vancomycin, colistin and nystatin. Vancomycin inhibits gram-positive bacteria. Colistin inhibits gram-negative bacteria and Nystatin inhibits fungi.
Modified Thayer-Martin Agar was later created when trimethoprim was added to the formula in order to inhibit Proteus species which was known to swarm plates.
CNA Agar
Columbia CNA Agar with 5% Sheep Blood is a selective and differential medium for the isolation and differentiation of gram-positive microorganisms.
CNA agar contains antibiotics Colistin and Nalidixic Acid which support the growth of gram-positive organisms like Staphylococci, Streptococci and Enterococci while inhibiting the growth of gram-negative organisms. This is done by disrupting their cell membranes and blocking their DNA replication.
Due to the addition of sheep blood, hemolysis can be observed on this agar, similar to that of TSA w/ 5% Sheep Blood Agar.
PEA Agar
Phenylethyl Alcohol (PEA) Agar is another selective medium for isolating gram-positive organisms, particularly gram-positive cocci. The selectiveness is due to the inhibition of DNA synthesis caused by the phenylethyl alcohol. 5% sheep blood is typically added, so hemolytic reactions can be seen, although they may be atypical.
Mannitol Salt Agar
Mannitol Salt Agar (MSA) is selective for Staphylococci in clinical specimens and is also differential for Staphylococcus aureus.
The 7.5% concentration of sodium chloride results in the partial or complete inhibition of organisms other than staphylococci, rendering it a selective media.
The fermentation of mannitol makes it a differential media. Coagulase positive staphylococci like Staphylococcus aureus will ferment mannitol and produce yellow colonies, whereas coagulase negative staphylococci will not ferment mannitol, therefore producing red colonies and no color change of the phenol red indicator.
Although Staph aureus is the main organism which ferments mannitol, there are lesser encountered species which also ferment the sugar like S. capitis, S. xylosus, S. cohnii, S. sciuri, S. simulans, etc. Because of this, further biochemical testing is necessary to confirm identification.
Macconkey Agar
The Macconkey Agar also known as MAC is a pinkish-red colored selective and differential media primarily used for stool cultures, although it is often used for most specimens like blood and chocolate agars.
Selective Media
This agar is supplemented with bile salts and crystal violet since they inhibit the growth of gram- positive organisms. This makes it “selective” for gram negative organisms, making it easier for identification of such bacteria. The most clinically significant bacteria like Enteric organisms like Escherichia coli, Shigella and Salmonella are all gram negative, making this a must have for a stool specimen workup.
Differential Media
Lactose is a carbohydrate which is added to help differentiate fermenters from non-fermenters on the same plate. If an organism like Shigella or Salmonella is not capable of fermenting lactose, the colonies will remain as colorless and is considered a Non-Lactose Fermenter (NLF). Whereas if an organism like Escherichia coli is able to ferment the lactose, a color indicator called neutral red will turn the colonies a pink-purplish color and is considered a Rapid Lactose Fermenter (RLF). This color change can help the microbiologist to further narrow down or differentiate between bacteria more easily.
MUG
MacConkey agar also has MUG (4-methylumbelliferyl β-D-glucuronide) added to it, which helps with identifying E. coli. Most strains of E. coli produce an enzyme which hydrolyzes MUG to yield a compound which fluoresces under long-wave (366 nm) UV light. When these strains of E.coli are present, they will fluoresce a blue-green color when examined under UV light.
Typical Colony Morphology on MacConkey Agar
Shigella: pink-colorless
Salmonella: pink-colorless
E.coli: pink-purplish
Hektoen Enteric Agar
Hektoen Agar also known as HEK is another selective and differential agar for the use of stool specimens. Hektoen Enteric Agar was developed in 1967 in order to improve the isolation of Shigella and Salmonella when compared with other media.
The selective nature of Hektoen Agar is due to its bile salts, which inhibit the growth of gram-positive organisms.
Hektoen Agar also contains the carbohydrates of lactose, sucrose and salicin. These assist with differentiation of organisms by virtue of their color change. Salmonella and Shigella are unable to utilize these sugars, therefore, they will remain a blueish-green color. Organisms like Escherichia coli which can ferment at least one of these sugars will turn an orange-salmon colored hue. The color indicators acid fuchsin and bromthymol blue are responsible for these color changes. Ferric ammonium citrate and sodium thiosulfate further enable differentiation by detecting Hydrogen Sulfide (H2S) production, resulting in black-centered colonies. Organisms like Salmonella will produce H2S gas, resulting in black colored colonies like these.
Typical Colony Morphology on Hektoen Agar
Shigella: blue-greenish
Salmonella: blue-greenish with black centers
E. coli: salmon-orange
TCBS Agar
Thiosulfate-Citrate-Bile-Sucrose Agar, also known as TCBS is a selective and differential media which is useful for isolating Vibrio cholerae and other Vibrio species, typically from stool specimens.
Vibrios are natural inhabitants of brackish and salt water worldwide. Human intestinal disease has been associated with consumption of contaminated water and shellfish or other seafood. Vibrio cholerae is the most commonly encountered species and causes severe diarrhea (cholera) known as “rice water stool” due to its visible similarities with pieces of rice. Other Vibrio species like V. alginolyticus, V. vulnificus, and V. damsela, are associated with extraintestinal infections, such as wound infections, septicemia and meningitis.
All Vibrio species that are pathogenic to humans, except for Vibrio hollisae, will grow on TCBS agar. TCBS has high concentrations of Sodium Chloride, which enhances Vibrio growth due to their halophilic or “salt loving” nature. Sodium citrate, sodium thiosulfate, oxgall, and cholate are added to provide an alkaline pH which inhibits gram-positive organisms, suppresses coliforms, and provides a pH environment more suitable to Vibrio growth.
Vibrios which ferment sucrose like Vibrio cholerae appear as yellow colonies. Vibrios which do not ferment sucrose including Vibrio parahaemolyticus, appear as green colonies.
Although TCBS agar is highly selective for Vibrio species, growth on this media should not be the sole source of identification. Further biochemical testing should be performed in order to confirm any suspected cases.
XLD Agar
XLD Agar is a selective and differential medium utilized for stool specimens.
It utilizes sodium desoxycholate as a selective agent causing growth inhibition of gram-positive organisms.
Xylose is incorporated into the medium since it is fermented by practically all Enterobacteriaceae except for Shigella. This lack of fermentation causes Shigella to have red colored colonies.
Lysine is included to enable Salmonella to be differentiated since without lysine.
Sodium Thiosulfate and Ferric Ammonium Citrate is added to help identify hydrogen sulfide (H2S) production. H2S producers will have growth resulting in the formation of black or black-centered colonies.
Colony Morphology on XLD Agar:
Salmonella- black or red colonies with black centers
Shigella- red colonies
E Coli- yellow colonies
EMB Agar
EMB Agar is a slightly selective and differential medium for isolating and differentiating gram-negative enteric bacilli.
EMB Agar contains Eosin Y and Methylene Blue dyes which slightly inhibit gram positive bacteria.
The dyes also serve as differential indicators in response to the fermentation of lactose and/or sucrose.
Coliforms (normal gut bacteria) produce blue-black colonies whereas Salmonella and Shigella colonies are colorless or have a transparent amber color. Escherichia coli colonies will grow as blue-black colonies but may also show a green metallic sheen due to the rapid fermentation of lactose.
Due to the slight selectiveness of the EMB agar, gram positive bacteria and yeasts may not be totally inhibited and will still be able to grow on the agar. In the cases where they do, they will usually be observed as pinpoint colonies.
Mueller Hinton Agar
Mueller Hinton Agar is a white to clear colored agar which is used for antimicrobial susceptibility testing. The “Kirby Bauer Test” is an example of this and is also commonly referred to as the Standardized Disc Diffusion Procedure. This test is performed on Mueller Hinton Agar and is commonly performed on patients in a clinical setting.
The Kirby-Bauer procedure starts off with an organism which has already been isolated and identified. This organism is mixed with a Mueller Hinton broth until the concentration is at a 0.5 McFarland standard. A swab is then dipped in the inoculate and spread across a Mueller Hinton Agar in three directions in order to achieve a “lawn of confluent growth”. Antibiotic impregnated discs are placed around the agar before it is then incubated overnight. When incubation is complete, the zone of inhibition around each disc is measured. If the bacteria grow all the way up to the disc, that organism would be labeled as R or “Resistant”. When there is a lack of growth seen as a “halo” around the disc this is considered S or “Susceptible”. When the zone of inhibition is in between that of resistant and susceptible, this is considered I or “Intermediate”. A ruler is used to measure the size of the zone of inhibition in millimeters (mm) around the disc and based off of CLSI performance standards, the interpretation of R, S or I is given for each individual antibiotic disc. These results save the doctor valuable time by identifying the most effective drugs towards an organism, instead of wasting time using less effective or even totally useless ones.
The Novobiocin Test is another use of Mueller Hinton agar, except instead of several antibiotic discs being placed around the agar, only one disc is needed (Novobiocin). This test is used for distinguishing between Staphylococcus species. S. saprophyticus is resistant to Novobiocin, allowing growth all the way up to the disc. Whereas with Coagulase-Negative Staph species like S. epidermidis will show a circular zone of inhibition around the disc.
Potato Dextrose Agar
Potato Dextrose Agar is used for cultivating yeasts and molds. Infusion from potatoes provides nutrition and dextrose helps stimulate growth. Tartaric acid is often added in order to reduce the pH in the agar, since it helps to inhibit bacterial growth.
Sabouraud Dextrose Agar
Sabouraud agar was developed to support the growth of fungi that cause skin, hair & nail infections, commonly known as dermatophytes. An acidic pH of pH 5.6 is used to inhibit bacterial growth. Additionally, some formulas will contain the antibiotic Chloramphenicol to further inhibit bacterial growth. Peptones like pancreatic digests of casein and meat peptones are sources of nutrition which enhance fungal growth, while dextrose supplementation provides energy. Unlike bacterial cultivation, fungi typically require long incubation periods up to 2-4 weeks or longer to achieve adequate growth. Common fungal organisms which are cultivated on Sabouraud Agar are Candida albicans, Cryptococcus neoformans and Trichophyton mentagrophytes.
Agar Slants
Agar slants, also known as agar slant tubes and nutrient agar slants are a type of agar which is inside of a glass tube. The tube is placed in a way so that the agar sets at an angle as it cools and hardens. This angle gives the tube an increased surface area for inoculation of organisms. There are multiple types of agar slants geared towards different purposes:
Triple Sugar Iron (TSI) Slant:
used for identifying gram negative enteric organisms. By observing the fermentation of sugars like dextrose, lactose and fructose and the production of gas and Hydrogen Sulfide (H2S), organisms like Salmonella, Shigella and E coli can be differentiated. When the sugar is fermented, the color will change from red to yellow. Non-fermentation will cause it stay a reddish color. Black precipitate signifies H2S production.
Urea Agar Slants:
used to differentiate gram negative bacteria based on urease reactivity. Organisms like Proteus mirabilis are rapid urease positive and will turn the urea agar from yellow to a pink color.
Incubation
Agar media must be incubated properly in order to achieve proper growth. There are 3 main components of this:
- Temperature: 20-24C, 37C and 42C.
- Time: can range from 24 hours up to multiple weeks
- Oxygen: Non-CO2, Increased CO2 and anaerobic conditions.

Case Studies
Given the above fundamentals, lets go over a few examples to demonstrate these principles.
Case Study 1: Wound Culture
Blood Agar: Growth
CNA Agar: Growth
MacConkey Agar: No Growth
In this example, there was growth on the CNA plate which tells us there is at least one gram positive organism in this wound. There was no growth noted on the MAC plate which tells us there are no gram negatives present. This would point to a gram positive like Staph being the likely organism.
Case Study 2: Urine Sample
Blood Agar: Growth
CNA Agar: No growth
MacConkey Agar: Growth (RLF)
In this example, the lack of growth on CNA agar lets us know we can rule out any gram-positive organisms. While the growth on MAC tells us that we have a gram negative. Additionally, the pink-purplish RLF colonies, let us know that we are encountering a rapid lactose fermenter like E. coli.
Conclusion

Agar media is vital to the field of laboratory medicine. It allows the microbiologist to identify pathogenic bacteria and fungi in a full range of specimen types. Growth on agar can be used to perform gram stains, observe colony morphology and perform biochemical testing, all of which are necessary components to confirm an organism identification.
References
BBL™ Columbia CNA Agar with 5% Sheep Blood and BBL™ MacConkey II Agar – I Plate™. (2017, November ). Retrieved from Becton Dickinson: https://www.bd.com/resource.aspx?idx=23018
BBL™ Modified Thayer-Martin (MTM II) Agar. (2007, September). Retrieved from Becton Dickinson: https://legacy.bd.com/europe/regulatory/Assets/IFU/US/L007392(09)(0907).pdf
BBL™ Trypticase™ Soy Agar with 5% Sheep Blood (TSA II) and BBL™ MacConkey II Agar with MUG–I Plate™. (2017, August). Retrieved from Becton Dickinson: https://www.bd.com/resource.aspx?IDX=23031
BBL™Chocolate II Agar (GC II Agar with Hemoglobin and IsoVitaleX™). (2007, September ). Retrieved from Becton Dickinson: https://www.bd.com/resource.aspx?IDX=31356
BD BBLä CHROMagarä Salmonella* / XLD Agar (Biplate) . (2013, March). Retrieved from Becton Dickinson: https://www.bd.com/resource.aspx?IDX=25504
BD Mueller Hinton II Agar. (2017, February). Retrieved from Becton Dickinson: https://www.bd.com/resource.aspx?IDX=8982
BDä EMB Agar (Eosin Methylene Blue Agar), Modified . (2013, March). Retrieved from Becton Dickinson: https://www.bd.com/resource.aspx?idx=8973
BDä Mannitol Salt Agar . (2013, March). Retrieved from Becton Dickinson: https://www.bd.com/resource.aspx?idx=8979#:~:text=BD%20Mannitol%20Salt%20Agar%20is,Staphylococcus%20aureus%20from%20clinical%20specimens
BD TCBS Agar. (2003, June). Retrieved from Becton Dickinson: https://legacy.bd.com/europe/regulatory/Assets/IFU/HB/CE/PA/PA-254432.pdf
Hare, J. (2008, September 8). Sabouraud Agar for Fungal Growth Protocols . Retrieved from American Society of Microbiology: https://asm.org/ASM/media/Protocol-Images/Sabouraud-Agar-for-Fungal-Growth-Protocols.pdf?ext=.pdf
Hektoen Enteric Agar. (n.d.). Retrieved from Becton Dickinson: https://legacy.bd.com/europe/regulatory/Assets/IFU/Difco_BBL/285320.pdf
Hektoen Enteric Agar Protocol . (n.d.). Retrieved from America Society for Microbiology: https://asm.org/ASM/media/Protocol-Images/Hektoen-Enteric-Agar-Protocol.pdf?ext=.pdf
Leeuwen, V., & Bladh. (2019). Davis’s Comprehensive Manual of Laboratory & Diagnostic Tests with Nursing Implications. In V. Leeuwen, & Bladh, Davis’s Comprehensive Manual of Laboratory & Diagnostic Tests with Nursing Implications (p. 373).
Phenylethyl Alcohol Agar; Phenylethyl Alcohol Agar with 5% Sheep Blood. (n.d.). Retrieved from Becton Dickinson: https://legacy.bd.com/europe/regulatory/assets/ifu/difco_bbl/211539.pdf
Potatoe Dextrose Agar. (2010, October ). Retrieved from Thermo Fisher: https://tools.thermofisher.com/content/sfs/manuals/IFU454311.pdf
Sabouraud Dextrose Agar pH 5.6 w/ and w/o Chloramphenicol. (2012, October 4). Retrieved from Fisher Scientific: https://assets.fishersci.com/TFS-Assets/LSG/manuals/IFU1768.pdf